Bioanalytical method development aims to achieve a clear definition of the design, the operating conditions, limitations of the bioanalytical method, and system suitability. The process ensures that the bioanalytical method being developed is up to the mark for undergoing its validation. Before bioanalytical method development, it is essential for the bioanalytical sponsors to obtain sufficient details regarding the detailed analyte study, including physicochemical characterization, in vivo and in vitro drug characterization, and studying the effects of the binding abilities of the protein.
Since times immemorial, the liquid chromatography-mass spectrometry (LCMS) technique has been widely employed throughout the pharmaceutical sector for analyzing the pharmaceutical drug candidate. Due to its precision and accuracy in bioanalytical quantification, the LCMS unit finds a wide array of application across the whole range of drug research and development process.
Analytical challenges in bioanalytical method development protocol –
The applicability of the LCMS for bioanalytical testing has been widely accepted as a promising analytical tool for the selection and sensitive determination of the pharmaceutical analytes of interest within a different and complex bioanalytical matrix.
For laboratory analyses, LC/MS/MS development strictly conforms to all the requirements as put forth under the regulatory environment. However, this poses a strict array of regulatory challenges along with ensuring that the method developed achieves the goal of its business of generating both the efficient and effective quality of results.
Understanding the challenges witnessed across the bioanalytical method development –
It is quite critical to obtain an appropriate protocol to achieve complete success in the bioanalytical method development protocol. Here is a glimpse of some of the challenges associated with the bioanalytical method development process –
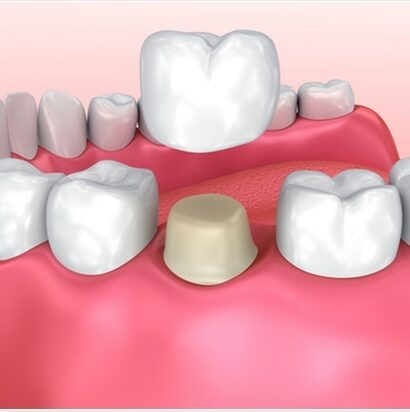
Matrix –
Biological matrices such as serum, urine, plasma, and tissues are routinely employed in the LCMS quantification, given that they possess different implications for facilitating the cleaning and accounting of the different interfering biological samples.
Number of sample analysis –
In an environment of high-throughput, the method suitability for automation is a critical factor to be considered.
Urgency –
Automated processes play a critical part in accomplishing a short turnaround time, basics of bioanalysis speed, and the scope of your bioanalytical method development process.
Number of analytes to be subjected to bioanalytical quantification –
The presence of a large number of analytes within the biological matrix will facilitate the determination of the analyte separation complexity, and the final scope of the method developed.
Required sensitivity level –
Arguably, the preliminary step for the detection and quantitation of the lower levels of your biological matrix’s analyte components becomes easily achievable with LCMS detection.
Troubleshooting the MS hardware for increasing the efficiency and reliability of the LCMS protocol –
The user friendly and intuitive tools supplementing the accessibility of the LCMS for bioanalysis ensures the following benefits –
Non-expert LCMS analysts can develop optimal MRM protocols with a certain degree of self-confidence, thereby facilitating the quantification process.
These new tools help in minimizing the overall time investment in the drug characterization, thereby assisting the experienced analysts in re-directing their time to attaining a higher expertise level.
The new user and intuitive tools help in providing consistency in utilizing the instrumentation and transferred information to GLP compliant software, thereby reducing the potential of possible errors.